Simple Synthesis of 4A Zeolite with the Addition of Al2O3 and Na2EDTA Compounds
DOI:
https://doi.org/10.55981/jsmi.2025.4302Keywords:
Zeolite synthesis, 4A zeolite, Non-magnetic fly ash, Fractionation, NaOH, Al2O3, Na2EDTAAbstract
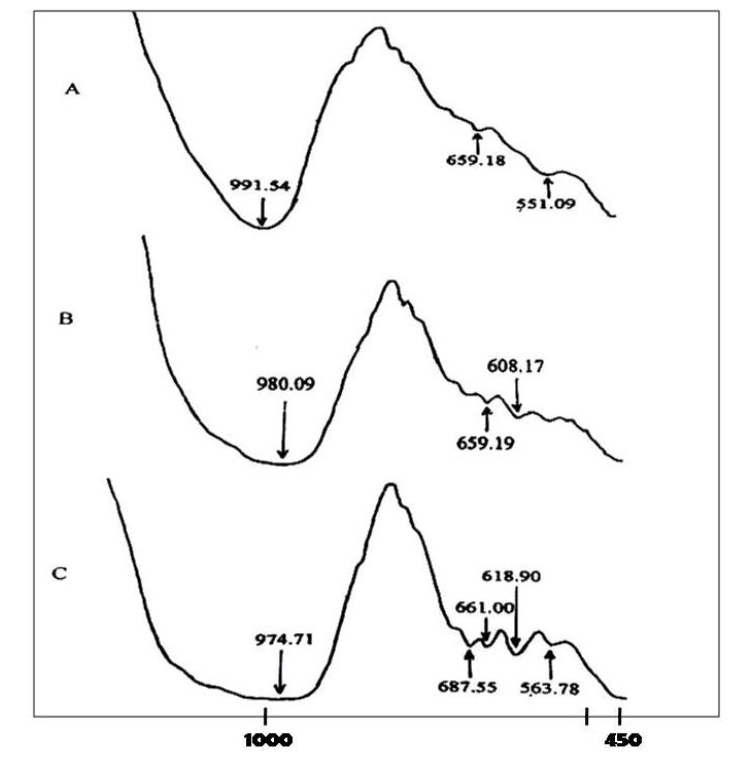
4A zeolite complex derived from coal has been successfully synthesized through the preparation of fractionated samples, analyzed using atomic absorption spectrophotometry (AAS), resulting in a chemical composition of silica (SiO2) 24.78% and aluminum oxide (Al2O3) 29.60%. The fly ash produced from this fractionation is used as the base material for the synthesis of 4A zeolite. The fly ash is reacted with sodium hydroxide (NaOH) at room temperature, yielding a gel that is subsequently crystallized into 4A zeolite through a reflux process. The resulting zeolite crystals are then supplemented with Al2O3 and disodium dihydrogen ethylenediaminetetraacetate (Na2EDTA) as sources of aluminum to achieve a molar ratio of Si/Al = 1.42. Optimal quality 4A zeolite crystals were obtained from the synthesis of 5.9351 g of medium fraction fly ash, along with 1 g Al2O3, 4 g NaOH, and 0.569 g Na2EDTA. Infrared (IR) characterization results indicate that the optimal 4A zeolite from medium fraction fly ash is characterized by crystals with the addition of 1.5 g of Al2O3. Wide absorption bandwidths are typically caused by large spectral line widths, which can occur if a significant amount of energy is absorbed by various vibrational modes, assuming that excess Al2O3 leads to the formation of functional groups that contribute to the same band. A peak at a wavelength of 564 cm-1 indicates double ring stretching vibrations, while a peak at 661 cm-1 indicates symmetric stretching vibrations of the zeolite framework. Meanwhile, peaks at wavelengths of 3460.86 cm-1 and 974.71 cm-1 indicate detected O-H absorption
Downloads
References
[1] Jumaeri, P. D. Jananti, and E. Kusumastuti. "Sintesis Zeolit A dari Abu Layang Batubara melalui Modifikasi Proses Hidrotermal." Sainteknol, vol. 11, no. 2, pp. 155-166, 2013.
[2] T. Las and H. Zamroni. "Penggunaan Zeolit Dalam Bidang Industri dan Lingkungan." Journal Zeolit Indonesia, vol. 1, no. 1, pp. 27-34, 2002.
[3] H. Prihastuti, Nuryoto, A. Irawan, and T. Kurniawan. "Pengaruh Penggunaan Asam terhadap Pemisahan Logam dari Abu Layang Batubara sebagai Bahan Dasar Sintesis Zeolit." J. Kartika Kimia, vol. 4, no. 1, pp. 13-20, 2021.
https://doi.org/10.26874/jkk.v4i1.72
[4] A. Iqbal, H. Sattar, R. Haider, and S. Munir. "Synthesis and Characterization of Pure Phase Zeolite 4A from Coal Fly Ash." Journal of Cleaner Production, vol. 219, pp. 258-267, 2019.
https://doi.org/10.1016/j.jclepro.2019.02.066
[5] L. Yang, X. Qian, P. Yuan, H. Bai, T. Miki, F. Men, H. Li, and T. Nagasaka. "Green Synthesis of Zeolite 4A Using Fly Ash Fused with Synergism of NaOH and Na2CO3." Journal of Cleaner Production, vol. 212, pp. 250-260, 2019.
https://doi.org/10.1016/j.jclepro.2018.11.259
[6] W. M. Putri, I. Purnamasari, M. Yerizam. "Sintesis Zeolit dari Bottom Ash Batubara untuk Menurunkan Kadar FFA pada Crude Palm Oil (CPO)." Jurnal Pendidikan Tambusai, vol. 7, no. 3, pp. 21792-21800, 2023.
[7] Syafriadi, S. Marhamah, and M. Al-Muttaqii. "Pengaruh Variasi Konsentrasi Naoh pada Zeolit Alam Lampung terhadap Produk Silika." Journal Riset Teknologi Industri, vol. 15, no. 2, pp. 393-402, 2021.
https://doi.org/10.26578/jrti.v15i2.6950
[8] Sudarno. "Pengaruh Komposisi NaOH pada konversi Abu Layang Batubara Paiton Menjadi Zeolit A, Sintesis dan Karakterisasi." Jurnal Penelitian Jurusan Kimia FMIPA, 2008.
[9] F. Bahmanzadegan, M. A. Pordsari, and A. Ghaemi. "Improving the efficiency of 4A zeolite synthesized from kaolin by amine functionalization for CO2 capture." Scientific Reports, no. 13, p. 12533, 2023.
https://doi.org/10.1038/s41598-023-39859-z
[10] C. Septommy and L. Badriyah. "Modifikasi Zeolit Alam Teraktivasi Asam dengan Perak Nitrat." Chemical Engineering Research Articles, vol. 5, no. 1, pp. 13-19, 2022.
https://doi.org/10.25273/cheesa.v5i1.10104.13-19
[11] Y. Cui, Y. Zheng, and W. Wang. "Synthesis of 4A Zeolite from Kaolinite-Type Pyrite Flotation Tailings (KPFT)." Minerals, vol. 8, no. 338, pp. 1-15, 2018.
https://doi.org/10.3390/min8080338
[12] N. Andarini, Z. Lutfia, and T. Haryati. "Sintesis Zeolit A dari Abu Terbang (Fly Ash) Batubara Variasi Rasio Molar Si/Al." Jurnal Ilmu Dasar, vol. 19, no. 2, pp 105-110, 2018.
https://doi.org/10.19184/jid.v19i2.5910
[13] S. Golbad, P. Khoshnoud, and N. Abu-Zahra. "Synthesis of 4A Zeolite and Characterization of Calcium and Silver-Exchanged Forms." Journal of Minerals and Materials Characterization and Engineering, vol. 5, no. 5, pp. 237-251, 2017.
https://doi.org/10.4236/jmmce.2017.55020
[14] Rismang, H. S. Syamsidar, R. Kurnia. "Sintesis Zeolit dari Abu Layang dengan Metode Hidrotermal dan Uji Adsorptivitas terhadap Logam Timbal (Pb)." Al-kimia, vol. 5, no. 2, pp. 127-135, 2017.
https://doi.org/10.24252/al-kimia.v5i2.3394
[15] A. E. Hidayat, S. S. Moersidik, and S. Adityosulindro. "Sintesis dan Karakterisasi Zeolit Hidroksi Sodalit dari Limbah Padat Abu Layang PLTU Batubara." Jurnal Ilmiah Teknik Sipil dan Teknik Kimia, vol. 4, no. 2, 85-91, 2019.
https://doi.org/10.33366/rekabuana.v4i2.1307
[16] T. Wahyuni. Suprapto, D. Prasetyoko. "Pengaruh Suhu Fusi terhadap Pembentukan Zeolit A dari Abu Layang Batubara Paiton: Kapasitas Penukar Kation (Ca2+)." Akta Kimindo, vol. 1, no. 1, pp. 42-51, 2016.
https://doi.org/10.12962/j25493736.v1i1.1429
[17] A. M. Khan and R. Singh. "Role of aluminum sources in the synthesis of zeolite X." Journal of Materials Science, vol. 55, no. 4, pp. 1426-1438, 2020.
[18] J. Feng and Y. Liu. "Synthesis of Zeolites in The Presence of Chelating Agents: A Comprehensive Study." Journal of Solid State Chemistry, vol. 289, pp. 121-129, 2021.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Januari Dawolo; Putra Hidayat Telaumbanua; O. Laia, N. K. Lase

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.