Characteristics of Phosphate Sorption on Surface Sediments: A Study in Kendari Bay
DOI:
https://doi.org/10.55981/limnotek.2024.2019Keywords:
phosphate adsorption, desorption kinetics, water-sediment interface, coastal development, Kendari BayAbstract
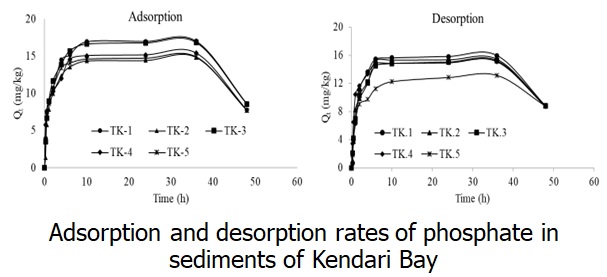
Phosphate adsorption and desorption are significant processes that influence the presence of phosphate in aquatic ecosystems and regulate the concentration of phosphate at the water-sediment interface. This research aims to investigate the characteristics of phosphate adsorption and desorption in Kendari Bay sediments, study the relationship between adsorption capacity and sediment characteristics and its phosphorus fraction, and evaluate its potential contribution to the overlying water column. Physicochemical measurements of the water and sediments were performed in the sampling location and the laboratory. Two types of adsorption-desorption kinetics models and two types of isothermal adsorption models were used to estimate the adsorption rate and capacity of the surface sediments. Adsorption kinetics and desorption kinetics experiments produced pseudo-second-order kinetic model equations with regression coefficients (R2) of 0.865–0.936 and 0.886–0.947, respectively. The isothermal adsorption experiment follows the Langmuir equation model with R2 = 0.964. The maximum adsorption capacity (Qmax) value was 156.3–227.3 mg/kg, and the phosphate concentration value at zero equilibrium (EPC0) was 0.0026–0.0047 mgP/L. Notably, the EPC0 value was higher than the SRP concentration, indicating that the resuspension of phosphate ions from sediment into the water column could occur. Furthermore, there was a correlation between Qmax values with OP, Al-P, Fe-P, clay particles, and organic materials. Potential practical applications may include integrating sediment adsorption capacity data into ecosystem models to inform nutrient management strategies and promote sustainable coastal development in Kendari Bay and beyond.
References
Al-Enezi E, Bockelmann-Evans B, Falconer R. 2016. Phosphorus adsorption/desorption processes of estuarine sediment: a case study—Loughor Estuary, UK. Arab J Geosci 9: 200.
Alfiani N, Salihin LMI, Usman I, Khairisa NH. 2019. Analisis Geospasial Sedimentasi Teluk Kendari Akibat Perkembangan Lahan Terbangun. Jurnal Geografi Aplikasi Dan Teknologi 3 (2):79.
Armid A, Shinjo R, Sabarwai SH, Ruslan R. 2017. Pollution Assessment of Various Heavy Metals in the Surface Sediment of Kendari Bay. Indonesia. J. Nature Environment and Pollution Technologi 16(4): 1067-1074.
Asriyana N, Irawati. 2019. Assessment of the trophic status in Kendari Bay. Indonesia: a case study. AACL Bioflux 12(2):650‒663.
Axinte O, Bădescu I, Stroe C, Neacsu V, Bulgariu L, Bulgariu D. 2015. Evolution of Trophic Parameters from Amara Lake. Environmental engineering and management journal. 14: 559-565.
Aydin I, Temel Z, Gunduzc B, Aydin F. 2018. Comparative determination of phosphorus fractionsin coastal surface sediment (NE Mediterranean Sea) by ICP-OES and UV/VIS Spectrometry. Atomic Spectroscopy. 39(5):193-197. http://doi.org/10.46770/AS.2018.05.003.
Barik SK, Bramh S, Bastia TK, Behera D, Mohanty PK, Rath P. 2019. Distribution of geochemical fractions of phosphorus and its ecological risk in sediment cores of a largest brackish water lake, South Asia. Int. J. Sediment Res. 34: 251–261.
Barik SK, Bramha S, Samanta S, Pattanaik AJ, Patel RK, Bastia TK, Samal RN, Behera D, Rath P. 2021. Phosphorus sorption behavior of the largest brackish water lagoon. South Asia. J Earth Syst Sci 130(48).
Bramha S, Mohanty AK, Padhi R, Panigrahi S, Satpathy KK. 2014. Phosphorus speciation in the marine sediment of Kalpakkam coast. southeast coast of India. Environmental Monitoring and Assessment. 186: 6003–6015.
Cao X, Liu X, Zhu J, Wang L, Liu S, Yang G. 2016. Characterization of phosphorus sorption on the sediments of Yangtze River Estuary and its adjacent areas. Mar. Pollut. Bull. 114(1):277–284.
CBSSS (Central Bureau of Statistics. Southeast Sulawesi). 2015. Southeast Sulawesi in Figures. Indonesia
Dim PE, Mustapha LS, Termtanun M, Okafor JO. 2021. Adsorption of chromium (VI) and iron (III) ions onto acidmodified kaolinite: Isotherm, kinetics and thermodynamics studies. Arabian Journal of Chemistry. 14(4): 103064. http://doi.org/ 10.1016/j.arabjc.2021.103064
El Semary NA. 2022. Iron-Marine Algal Interactions and Impacts: Decreasing Global Warming by Increasing Algal Biomass. J. Sustainability. 14: 10372. http://doi.org/10.3390/ su141610372
Eviati and Sulaeman. 2009. Analisis Kimia Tanah, Tanaman, Air, dan Pupuk. In: Prasetyo. B.H.. Santoso D.. Retno. W.L. editor. Petunjuk Teknis. Balai Penelitian Tanah. 2: 234 pages.
Ghaisas NA, Maiti K, White JR. 2019. Coupled iron and phosphorus release from seasonally hypoxic Louisiana shelf sediment. J. Estuaine, Coastal, and Shelf Science 219: 81–89.
Gerard F. 2016. Clay minerals. iron/aluminum oxides. and their contribution to phosphate sorption in soils – a myth revisited. Geoderma. 262: 213–226
Guo CH, Li HX, Fang F, Ji YS, Xing YX, Fan YB, Liu Y. 2018. Study on distribution of phosphorus fractions and dsorptiondesorptioncharacteristics in surface sediments of the Yellow River by molybdenum antimony spectrophotometry. Spectrosc Spectr. Anal. 38(1):218–223
Ho YS dan McKay G. 1999. Pseudo-second order model for sorption processes Process Biochem. 34(5):451-465. https://doi.org/10.1016/S0032-9592(98)00112-5.
Hou A, Chen P, Alloatti J, Mozzoni L, Zhang B, Shi A. 2009. Genetic variability of seed sugar content in worldwide soybean germplasm collections. Crop Sci 49:903–912.
Huang WY, Li D, Liu Z, Tao Q, Zhu Y, Yang J, Zang YM. 2014. Kinetics. Isotherm. Thermodynamic. and Adsorption Mechanism of La(OH)3-modified Exfoliated Vermiculites as Highly Efficient Phosphate Adsorbents. Chemnical Engineering J 236: 191-201.
Jiang Y, Ma XL, Wang B, Jiang BB, Wang WK, Wang YJ, Zhang CD. 2022: Effects of environmental factors on phosphorus adsorption capacity and release risk in lake sediments. Plant Soil Environ. 68: 186–194
Kang X, Song J, Yuan H, Shi X, Yang W, Li X, Li N, Duan L. 2017. Phosphorus speciation and its bioavailability in sediments of the Jiaozhou Bay. Estuarine, Coastal, and Shelf Science 188: 127–36.
Khamizov RK. 2020. A Pseudo-Second Order Kinetic Equation for Sorption Processes. Russ. J. Phys. Chem. 94:171–176. http://doi.org/10.1134/S0036024420010148
Larasati A, Notoatmodjo S. 2014. Equilibrium and kinetics of orthophosphate removal from aqueous phase with adsorption-desorption methods. J. Teknik Lingkungan. 20(1): 38-47.
Li J, Reardon P, McKinley JP, Joshi SR, Bai Y, Bear K, Jaisi DP. 2017. Water column particulate matter: A key contributor to phosphorus regeneration in a coastal eutrophic environment, Chesapeake Bay. J. Geophys. Res. Biogeosciences. 122: 737–752. http://doi.org/10.1002/2016JG003572
Li P, Zhao C, Liu K, Xiao X, Wang Y, Wang Y, He D. 2021. Anthropogenic Influences on Dissolved Organic Matter in Three Coastal Bays, North China. Journal Frontiers in Earth Science: section Biogeoscience. 9: 697758. http://doi.org/10.3389/feart.2021.697758
Makkawaru A, Sideng U, Sufrianto. 2021. Interpretation of the Geological Romance of Kendari Bay Using Landsat 5 TM. LaGeografia 20:114.
Maslukah L, Wulandari SY, Yasrida A. 2017. Rasio Organik Karbon Terhadap Fosfor Dalam Sedimen Di Muara Sungai Banjir Kanal Barat. Semarang. Buletin Oseanografi Marina 6(1): 39-45.
Maslukah L, Sugianto D, Salma U, Zainuri M, 2019. Phosphorus Fractionation and Its Bioavailability in Panjang Island Jepara. IOP Conference Series: Earth and Environmental Science 246: 012051.
Maslukah L, Zainuri M, Wirasatriya A, Widiaratih R. 2020. Studi Kinetika Adsorpsi dan desorpsi Ion Fosfat (PO42-) di Sedimen Perairan Semarang dan Jepara. J. Ilmu dan Teknologi Kelautan Tropis 12(2): 383-394.
Meng J. Yao Q, Yu Z. 2014. Particulate phosphorus speciation and phosphate adsorption characteristics associated with sediment grain size. Ecological Engineering 70: 140–145.
Meng J, Yu Z, Yao Q, Bianchi TS, Paytan A. Zhao, B, Pan H, Yao P. 2015. Distribution, mixing behavior, and transformation of dissolved inorganic phosphorus and suspended particulate phosphorus along a salinity gradient in the Changjiang Estuary. Marine Chemistry, 168 : 124–134. http://doi.org/ 10.1016/ j. marchem.2014.09.016
Mucha AP, Vasconcelus MTSD, Bordalo AA. 2003. Macrobenthic community in the Douro Estuary relation with trace elements and natural sediment Characteristic. Environmental Pollution 12(2): 16-180
Omari H, Dehbi A, Lammini A, Abdallaoui A, 2019. Study of the Phosphorus Adsorption on the Sediments. Journal of Chemistry. 10 pages
Putra I, Mihardja DK, Trismadi T, Pranowo WS, Lazuardi R, Nugroho PE. 2021. Analisi Ketidaksimetrisan Pasang Surut Akibat Pengaruh Morfologi di Teluk Kendari: Study of Tidal Asymmetry as The Morfological Effect in Kendari Coastal Bay. Jurnal Chart Datum. 7(2):73–86. https://doi.org/10.37875/chartdatum.v7i2.210
Rout, P.R., P. Bhunia, RR. Dash. 2014. Modeling isotherms. kinetics. and understanding the mechanism of phosphate adsorption onto a solid waste: ground burn patties. J. of Environmental Chemical Engineering. 2(3): 1331-1342.
Simanjuntak, N., Rifardi, A. Tanjung. 2020. Hubungan Karakteristik Sedimen dan Bahan Organik Sedimen dengan Kelimpahan Kerang Darah (Anadara granosa) di Perairan Tanjung Balai Asahan ProvinsiSumatera Utara. J. Perikanan dan Kelautan. 25(1): 6-17
Tu L, Jarosch KA, Schneider T, Grosjean M. 2019. Phosphorus fractions in sediments and their relevance for historical lake eutrophication in the Ponte Tresa basin (Lake Lugano, Switzerland) since 1959. Science of the Total Environment. 685:806-817.
U.S. EPA. 1996. METHOD 3050B. U.S. Environmental Protection Agency. Washington. D.C.. EPA 600/P-./001.
Van Diggelen JMH, Lamers LPM, Van Dijk G, Schaafsma MJ, Roelofs JGM, Smolders AJP. 2014. New insights into phosphorus mobilization from sulfur-rich sediments: time-dependent effects of salinization. PLoS One 9(11): e111106.
Veerasingam S dan Venkatachalapathy R. 2014. Estimation of carbonate concentration and characterization of marine sediments by Fourier Transform Infrared Spectroscopy. Infrared Physics & Technology. 66:136–140. http://doi.org/10.1016/j.infrared.2014.06.0
Vicente MAF, Melo GVD, Neto JAB, Oliveira ASD. 2016. Phosphorus Fractionation Distribution in Guapimirim estuary: SE Brazil. SpringerPlus. 5:1406. http://doi.org/ 10.1186/s40064-016- 3065-9
Wang LQ, Liang T, Chen Y. 2015. Distribution characteristics of phosphorus in the sediments and overlying water of Poyang Lake. Plos One 10(5): e0125859.
Wang X, Wei J, Ba N, Cha H, Cao C, Zheng K, Liu Y. 2018. The phosphorus fractions and adsorption-desorption characteristics in the Wuliangsuhai Lake. China. Environ Sci Pollut Res 25: 20648–20661.
Yan Y, Gao B, Hao H, Zhou HD, Lu J. 2017. Nitrogen and phosphorus in sediments in China: A national-scale assessment and review. Sci. Total Environ. 576: 840–849
Yang B, Liu S, Zhang G. 2018. Geochemical Characteristics of Phosphorus In Surface Sediments From The Continental Shelf Region Of The Northern South China Sea. Marine Chemistry. 198: 44–55. https://doi.org/10.1016/j.marchem.2017.11.001
Yang, X., X. Chen, X. Yang. 2019. Effect of organic matter on phosphorus adsorption and desorption in black soil from Northeast China. Soil and Tillage Research. 187. 85-91.
Yilmaz E, Koc C. 2012. A Study On Seasonal Changes Of Phosphorus Fractions In Marine ¨ Kova Bay. Turkey Sediments of The Akyaka Beach in Gökova Bay, Turkey. Clean Technologies and Environmental Policy 14(2): 299–307.
Zhang WL, Zeng CS, Tong C, Zhai SJ, Lin X, Gao DZ. 2015. Spatial distribution of phosphorus speciation in marsh sediments along a hydrologic gradient in a subtropical estuarine wetland, China. Estuar Coast Shelf Sci154:30–38.
Zheng Z, Wang X, Jin J, Hao J, Nie Y, Chen X, Mou J, Emslie SD, Xiaodong L. 2022. Fraction distribution and dynamic cycling of phosphorus in lacustrine sediment at Inexpressible Island, Antarctica. Environ. Int. 164:107228. http://doi.org/10.1016/j.envint.2022.107228
Zhuang W, Gao X, Zhang Y, Xing QG, Tosi L, Qin S. 2014. Geochemical characteristics of phosphorus in surface sediments of two major Chinese mariculture areas: The Laizhou Bay and the coastal waters of Zhangzi Island. Mar. Pollut. Bull 83: 343–351
Zhu H, Wang D, Cheng P, Zhong JBC. 2015. Effects of sediment physical properties on the phosphorus release in aquatic environment. Sci. China Phys. Mech. Astron. 58. 1–8
Zulham A, Subaryono, Mahulette. 2017. Pengembangan Perikanan Tangkap Laut Kota Kendari. Wudianto, Baskoro M, Wijopriono (Eds). : Rajawali Pers, 2017. xiv, 66 pages.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Authors

This work is licensed under a Creative Commons Attribution 4.0 International License.






